Severe Acute Respiratory Syndrome Review and Lessons of the 2003 Outbreak
Abstruse
Severe acute respiratory syndrome (SARS) was caused by a previously unrecognized animal coronavirus that exploited opportunities provided past 'wet markets' in southern Red china to adapt to become a virus readily transmissible between humans. Hospitals and international travel proved to be 'amplifiers' that permitted a local outbreak to reach global dimensions. In this review we volition discuss the substantial scientific progress that has been made towards understanding the virus—SARS coronavirus (SARS-CoV)—and the disease. We will also highlight the progress that has been made towards developing vaccines and therapies The concerted and coordinated response that independent SARS is a triumph for global public wellness and provides a new prototype for the detection and control of hereafter emerging communicable diseases threats.
Main
The past 150 years saw the emergence of three pandemics from southern China: plague during the belatedly nineteenth century and ii influenza pandemics (Asian flu of 1957 and Hong Kong flu of 1968)1,2. In Nov 2002, a new 'plague' was emerging in Guangdong Province, Mainland china. On 21 February 2003 a physician from Guangdong spent a single day in hotel 'M' in Hong Kong, during which time he transmitted an infection to 16 other guests. These, in plough, seeded outbreaks of the disease in Hong Kong, Toronto, Singapore and Vietnam3. Within weeks, SARS had spread to affect more than 8,000 people in 25 countries beyond 5 continents (Fig. 1; Earth Health Organization, http://www.who.int/csr/sars/land/table2004_04_21/ en_21/en/impress.html). Past the stop of the global outbreak (5 July 2003), information technology had killed 774 people—a pocket-sized number in comparison with the fatalities during the previous pandemics of plague and influenza. Merely the rapidity of spread past air travel, firsthand media coverage and today'southward globalization of economic activity all contributed to the far more pronounced impact of SARS.
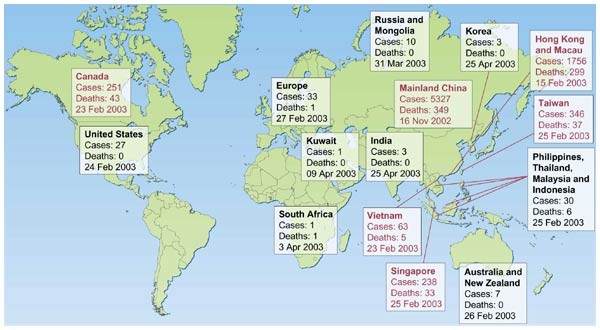
The number of likely cases of SARS and the date of onset of the beginning instance in each country (or group of countries) is denoted. The countries denoted in ruby are those where substantial local manual occurred. The data are based on World Wellness System, http://www.who.int/csr/sars/country/ table2004_04_21/en_21/en/print.html and the effigy is adapted from ref. 15.
The speed of the scientific response in understanding this new viral affliction was unparalleled. The clinical syndrome was described4,five,6, the etiological amanuensis identifiedseven,8,nine, diagnostic tests devised9,10 and the genome completely sequencedxi,12 within weeks of the virus's emergence from mainland People's republic of china. Simply ane.five years later, the start stage i vaccine trials are underway, and several other vaccine candidates are under evaluation in brute modelsthirteen. Previous reviews have addressed aspects of the clinical presentation14,fifteen,16, etiology17, virology18,19,twenty, laboratory diagnosis21, epidemiology (ref. 22 and Earth Health Organization, http://www.who.int/csr/sars/en/whoconsensus.pdf), infection command, clinical direction and public health23,24,25. Hither we emphasize aspects of pathogenesis and their correlation to clinical upshot, and discuss the progress that has been made towards antiviral treatment and vaccine development.
The virus, its origins and development
SARS probably first emerged in Guangdong around November 2002 (refs. 26,27). Many of the affected individuals in November and Dec 2002 had contact with the live-game trade27. The disease was described equally an "infectious atypical pneumonia" considering of its propensity to cause clusters of affliction in families and healthcare workers28. The etiological agent of SARS was identified as a new coronavirus not previously endemic in humans7,eight,9. The lack of serological prove of previous infection in salubrious humans suggested that SARS-CoV had recently emerged in the human population and that beast-to-human interspecies transmission seemed the near probable explanation for its emergence. Specimens collected from apparently healthy animals (e.thousand., Himalayan palm civets (Paguma larvata) and raccoon dogs (Nyctereutes procyonoides)) plant in alive wild-game beast markets in Guangdong yielded a SARS-CoV-similar virus with more than 99% nucleotide homology to the homo SARS-CoV29. Just the wild-beast reservoir in nature still has not been identified conclusively. Many workers who handled animals in these wet markets had antibody to the related animal SARS-CoV-like virus although they had no history of a SARS-like affliction29,thirty. Taken together with the observation that a number of the SARS-affected individuals in November and December 2002 had epidemiological links to the wild-game animal trade27, it is likely that these wet markets in Guangdong (Fig. 2) provided the interface for transmission to humans. The early interspecies transmissions to humans were probably inefficient, causing piddling human disease or transmission between humans. Eventually, the fauna precursor SARS-CoV-similar virus probably adapted to more efficient human-to-human transmission, and SARS emerged. As ii authors aptly stated, this was "i modest footstep to man, one giant leap to mankind"31.

(AP Photo/Xinhua, Liu Dawei)
In some regions (e.g., Guangdong province, Red china), increasing affluence has led to the proliferation of markets housing a range of live 'wild' animal species, such equally civet cats, pictured, linked to the restaurant trade servicing the demand for these exotic foods.
The new coronavirus associated with SARS (SARS-CoV) is phylogenetically distinct from all previously known human and brute coronaviruses11,12,32,33. There is also show that SARS-CoV evolved towards greater 'fitness' in the human host during the class of the outbreak. Compared with animal SARS-similar viruses and early human SARS-CoV strains, human being viruses isolated later during the outbreak had acquired a 29- (in some, a 415-) nucleotide deletion in open up reading frame (ORF)8 (refs. 29,34). The biological significance of these deletions, nonetheless, is not clear. Similarly, SARS-CoV in individuals before February 2003 was genetically more diverse than the after isolates26,34,35. The fasten protein (the viral surface glycoprotein which mediates viral attachment and entry into the prison cell; Fig. three) of early isolates contained higher rates of nonsynonymous mutations, probably reflecting the ongoing adaptation to the new host. The relative genetic homogeneity of SARS-CoV isolates from afterwards in the outbreak34,35,36,37 may reverberate a virus better adapted to the new host. The fact that much of the global spread arose from one index example in Hotel M in Hong Kong3,35 may also contribute to this genetic homogeneity.
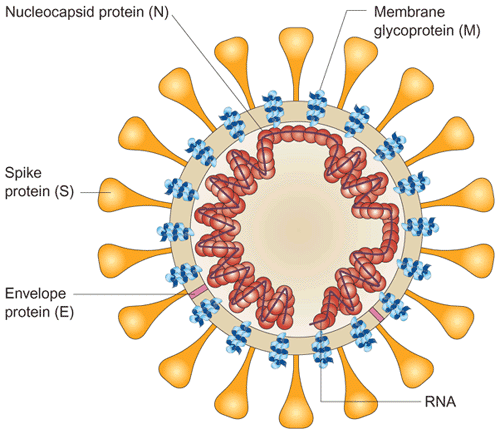
The viral surface proteins (spike, envelope and membrane) are embedded in a lipid bilayer envelope derived from the host cell. Unlike group 2 coronaviruses, SARS-CoV does not possess a hemagglutinin esterase glycoprotein. The single-stranded positive-sense viral RNA is associated with the nucleocapsid protein.
A ban on the auction of wildlife in moisture markets in Guangdong imposed during the later menstruum of the SARS outbreak was lifted in September 2003. Between 16 December and thirty January 2004, at that place were 4 new cases of SARS, the commencement nonlaboratory-associated cases diagnosed in humans since the terminate of the SARS outbreak in July 2003. Epidemiological linkage and phylogenetic information suggest that the associated viruses were new introductions from animals (Y. Guan, unpublished observations)34,38,39. These human cases were relatively mild and did non lead to secondary transmission, reflecting that the animal precursor virus is probably not well adapted to efficient human-to-human being transmission. This is probably a recapitulation of events in late 2002 in the run-upward to the SARS outbreak in 2003. This fourth dimension, the findings led to the reintroduction of the ban on wild-game animal markets and there have been no farther naturally caused human cases since.
It is probable that the forerunner of SARS-CoV has repeatedly crossed the species barrier but but occasionally has it succeeded in adapting to human–human transmission. This adaptation clearly occurred in belatedly 2002 and it may happen once more in the future. But given the present understanding and awareness about SARS, we expect that such re-emergence is unlikely to lead to a global outbreak on the scale of 2003.
Transmission between humans
The major routes of transmission of SARS are droplet infection, aerosolization and fomites (refs. xl,41 and Globe Health Organization, http://www.who.int/csr/sars/en/whoconsensus.pdf). Degradation of infected droplets or aerosols on the respiratory mucosal epithelium probably initiates viral infection. Whether infection can occur through the oral or conjunctival routes is unknown, but SARS-CoV has been detected in tears42. Although exposure to the animal precursor of SARS-CoV seems to have resulted in asymptomatic infection29,30, once the virus had adapted to man-to-human being transmission in the afterward part of the outbreak, asymptomatic infection seemed to be rare43. Other peculiarities virtually SARS-CoV transmission were also evident. Transmission was infrequent during the first 5 days of disease44 and, unlike transmission of influenza, was relatively inefficient in the household setting45. Despite SARS's fearsome reputation and global spread, the average number of secondary infectious cases generated by ane case (R 0) was depression (two.2–iii.7); in contrast, the R 0 of influenza ranges from 5 to 25 (ref. 22). Although not unique to SARS, 'superspreading events' (in which a few affected individuals disproportionately contribute to manual) were characteristic of the outbreak22,46. The factors underlying the superspreading phenomenon of SARS are poorly understood but may include coinfection with other viruses and host factors, as well as behavioral and environmental factors.
Clinical symptoms
The clinical symptoms of SARS-CoV infection are those of lower respiratory tract disease4,5,6,vii,14. Besides fever, malaise and lymphopenia, afflicted individuals have slightly decreased platelet counts, prolonged coagulation profiles and mildly elevated serum hepatic enzymes. Chest radiography reveals infiltrates with subpleural consolidation or 'ground glass' changes compatible with viral pneumonitis. Only although the primary clinical symptoms are those of astringent respiratory disease, SARS-CoV actually causes infection of other organs: some afflicted individuals have watery diarrhea, and virus can exist cultured from the feces and urine, also equally the respiratory tract47,48,49. In addition, RT–PCR has identified the virus in the serum, plasma and peripheral claret leucocytesl,51. Individuals with SARS likewise have a pronounced peripheral T-cell lymphocytopenia: numbers of CD4+ and CD8+ cells are both reduced, and more than than i-third of individuals have a CD4+ T-cell count of less than 200 cells/mm3 (refs. 52,53), suggesting increased susceptibility to secondary infections. The mechanisms underlying the T-cell lymphopenia remain to be elucidated.
Around twenty–30% of individuals with SARS crave direction in intensive care units14 and the overall fatality charge per unit is ∼15% (World Health Organization, http://www.who.int/csr/sars/en/whoconsensus.pdf). The age dependence of disease severity and mortality is notable; during the outbreak, mortality rates of affected individuals in Hong Kong who were 0–24, 25–44, 45–64 and >65-year old were 0%, 6%, 15% and 52%, respectively (World Wellness System, http://www.who.int/csr/sars/en/WHOconsensus.pdf). None of the 1–12-twelvemonth-olds infected with SARS-CoV in Hong Kong had disease astringent enough to require intensive care or mechanical ventilation54,55. This progressive historic period dependence in mortality is not totally explained by comorbid factors and the underlying biological basis remains unclear.
Virus tropism and pathogenesis
Quantitative studies of viral load have provided insights into the pathogenesis of SARS. Viral load is higher in the lower respiratory tract than in the upper airways56,57. Viral load in the upper respiratory tract47 and feces57 is depression during the showtime 4 days and peaks at around twenty-four hours ten of affliction. In marked dissimilarity, viral load in flu peaks shortly after onset of clinical symptoms58. This unusual feature of SARS-CoV infection explains its low transmissibility early in the illness. It as well explains the poor diagnostic sensitivity of the first-generation RT–PCR diagnostic tests on upper respiratory tract and fecal specimens collected early on in the illness (reviewed in ref. 21).
Afflicted individuals with high serum viral loads have a poor prognosis59. Between days ten–fifteen of disease, high viral load in nasopharyngeal aspirates, feces and serum, as well every bit detection of virus in multiple anatomic sites, are independently predictive of adverse clinical outcome60. Series studies of viral load throughout illness also reflect clinical outcome61. Taken together, these findings suggest that poor clinical consequence is associated with continued uncontrolled viral replication. SARS-CoV RNA tin can be invariably detected in the lungs of individuals dying of SARS, but viral load is college in those dying earlier in the course of the disease (<21 days)62.
The respiratory tracts of afflicted individuals who dice during the first ten days of illness prove diffuse alveolar damage with a mixed alveolar infiltrate, lung edema and hyaline membrane formation. Macrophages are a prominent component of the cellular exudates in the alveoli and lung interstitium63,64. Multinucleate syncytia of macrophage or epithelial cell origin are sometimes seen afterward in the disease. Immunohistochemistry, in situ hybridization and electron microscopy on autopsy or tissue biopsy have unequivocally demonstrated SARS-CoV replication in pneumocytes in the lung and enterocytes in the intestine65,66,67,68. Individual reports of virus detection by in situ hybridization or immunohistochemistry in other tissues69 await confirmation past electron microscopyseventy.
In the large and minor intestines, the virus replicates in enterocytes71. Viral particles primarily are seen on the apical surface of enterocytes and rarely in the glandular epithelial cells. Simply there is no villous cloudburst or cellular infiltrate in the abdominal epithelium and the pathogenic mechanisms responsible for watery diarrhea in individuals with SARS is unclear. Some human intestinal epithelial cell lines support productive replication of SARS-CoV72 and cistron expression arrays have shown that virus replication is associated with the expression of an antiapoptotic host cellular response, peradventure explaining the lack of enterocyte destruction in vivo 73.
The virus receptor and entry into cells
Studies using pseudotyped lentiviruses, carrying the spike, membrane and envelope surface glycoproteins of SARS-CoV (Fig. 3) separately and in combination demonstrated that the spike protein is both necessary and sufficient for virus attachment on susceptible cells74,75,76,77. The SARS-CoV fasten protein uses a mechanism similar to that of class ane fusion proteins in mediating membrane fusion78,79. There is no consensus every bit to whether the virus entry occurs through a pH-dependent receptor-mediated endocytosis or through direct membrane fusion at the cell surface74,77,eighty. The receptor for SARS-CoV was identified every bit the metallopeptidase ACE-two (refs. 81,82). The soluble ACE-2 ectodomain blocks SARS-CoV infection76, and amino acids 270–510 of the fasten protein are required for interaction with ACE-2 (ref. 83). Other coronaviruses use different cell receptors and enter cells either by means of fusion at the plasma membrane or through receptor-mediated endocytosis84.
Immunostaining techniques have identified ACE-2 on the surface of type 1 and 2 pneumocytes, the enterocytes of all parts of the small-scale intestine and the proximal tubular cells of the kidney. This localization explains the documented tissue tropism of SARS-CoV for the lung and alimentary canal and its isolation from the urine. Just information technology is notable that colonic enterocytes lack ACE-2 protein expression although SARS-CoV replication does occur in colonic epithelium71,85. In contrast, whereas ACE-2 is strongly expressed on the endothelial cells of small and big arteries and veins of all tissues studied and the smooth muscle cells of the intestinal tract, there is no evidence of virus infection at any of these sites. This lack of virus infection in tissues that express the putative receptor prompts the question of whether a coreceptor is required for successful virus infectionlxx. Vasculitis is known to occur in individuals with SARS simply its relation to infection of endothelial cells is unknown. Because only the basal layer of the nonkeratinized squamous epithelium of the upper respiratory tract expresses ACE-two (ref. 85), undamaged epithelium of the nasopharynx is unlikely to support SARS-CoV replication. Other receptors for virus entry that are independent of ACE-2 expression may exist.
Pseudotyped virus containing the spike poly peptide has besides been shown to bind to dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)74. DC-SIGN is a type-II transmembrane adhesion molecule institute on dendritic cells consisting of a C-type lectin domain that recognizes saccharide residues on a diverseness of pathogens. Dissimilar the ACE-2 receptor on pneumocytes and enterocytes, DC-SIGN does not permit SARS-CoV infection of the dendritic cells. Instead, binding of SARS-CoV to DC-SIGN allows dendritic cells to transfer infectious SARS-CoV to susceptible target cells74. A similar mechanism has been described for dengue virus, human being immune deficiency virus (HIV) and cytomegalovirus, and may be relevant in SARS pathogenesis.
Many details of SARS-CoV pathogenesis remain to be elucidated, but the evolution of a total-length infectious cDNA clone of SARS-CoV should permit precise manipulation of the virus genome and volition help our understanding of the viral determinants of pathogenesis86.
The host response
Several inflammatory cytokines (IL-1β, IL-half-dozen and IL-12) and chemokines chemotactic for monocytes (MCP-i) and neutrophils (IP-x) are elevated in adults and children with SARS87,88,89,90. The increased levels of monocyte-tropic chemokines may contribute to the prominently monocytic macrophagic infiltrate observed in the lung63. Only increases of these aforementioned chemokines occur in other viral diseases (due east.one thousand., influenza)91 and are non a unique feature of SARS. In addition, ELISPOT assays of peripheral blood leukocytes take revealed prolonged immunological dysregulation in individuals with SARS92. It is difficult to evaluate the overall pathogenic significance of these findings considering immunological markers in the peripheral claret practise not e'er reflect the local microenvironment of the lung93.
Genetic factors associated with susceptibility to, or severity of, SARS are under investigation. HLA-B*4601 has been associated with severe SARS disease in Taiwan94 just non Hong Kong95. HLA-B*0703 has also been associated with disease susceptibility and HLA-DRB1*0301 with resistance to SARS. The coinheritance of B*0703 and B60 was significantly higher in individuals with SARS than in the general population95. The mechanisms underlying these disease associations remain to be elucidated.
Animal models
Key to the evolution of effective antiviral drugs and vaccines confronting SARS-CoV was the development of animal models of SARS (Table one). SARS-CoV seems to cause infection in cynomolgous macaques following intratracheal inoculation96,97,98. Merely whereas some researchers find prove of disease pathology reminiscent of that seen in individuals dying of SARS and can show SARS-CoV antigen and viral particles in the pneumocytes of infected macaques96,97, others only find evidence of a mild upper-airway affliction and depression levels of virus by RT-PCR98. These differences in outcome may reflect differences in the viral strain, pre-exposure history and age of the animals, route of inoculation, phase of infection at which necropsy was performed or other factors.
Other animal models include ferrets, cats, Aureate Syrian hamsters, mice and African dark-green monkeys (Table one)99,100,101,102,103. These animal models support viral replication in the upper and lower respiratory tracts96,97,98,99,100,101,102,103. Ferrets and hamsters also develop notable lung pathology. Infected cats and ferrets transmit SARS-CoV to noninfected animals held in the same cage99. Natural asymptomatic infection in cats was documented during the community outbreak at Amoy Gardens, Hong Kong (World Health Organisation, http://www.who.int/csr/sars/en/whoconsensus.pdf).
These beast models of SARS differ from natural human disease in that the period between infection and peak disease pathology or acme viral load is shorter than is establish in man disease and because the affliction pathology, when present, is cocky-limited and rarely progresses to a fatal outcome as occurs with SARS. They likewise do non accurately reproduce the intestinal component of the human disease. But these models provide the only options presently available for addressing questions relevant to therapeutics and vaccine development. They can provide useful data providing their limitations are recognized.
Antiviral therapy
Several potential antiviral agents have been evaluated in vitro, and a few take been tested in animal models. Screening of currently available antiviral drugs and chemical libraries reveals that interferons, glycyrrhizin, baicalin, reserpine, niclosamide, luteolin, tetra-O-galloyl-β-D-glucose and the protease inhibitors have in vitro activity against SARS-CoV104,105,106,107,108. Differences in in vitro susceptibility of SARS-CoV to interferon (IFN)-β1b, IFN-α2 and ribavirin106,109,110,111 probably relate to differences in the testing methods used. Overall, IFN-αn1/n3, leukocytic IFN-α, IFN-β and the HIV protease inhibitors (particularly nelfinavir) are consistently active in vitro and should be considered for animal studies and randomized placebo-controlled clinical trials. Type i interferons render uninfected cells refractory to SARS-CoV replication through a MxA-independent machinery112, whereas the HIV protease inhibitors may cake the activeness of the main SARS-CoV proteinase113. So far, only interferons accept been tested in animal models: in cynomolgous macaques, pegylated IFN-αn2 provided prophylaxis merely was merely marginally effective for early treatment114. No randomized placebo-controlled trials have been performed for any of these antiviral drugs, although treatment studies using historical controls have suggested clinical benefit from IFN-α (infacon-1)115 and the combination of a protease inhibitor with ribavirin61.
The rapidity with which the SARS-CoV genome was sequenced, the determination of the structure of potential drug targets116 and the prediction of functional properties of SARS-CoV proteins based on prior cognition of homologs from other coronaviruses117 have immune identification of potential new drug targets. Peptides derived from the heptad-repeat-2 region of the spike protein accept been shown to block virus infection, admitting at much college molar concentrations than similar inhibitors needed to prevent HIV entry78,79. Short interfering RNAs also seems to be effective in decreasing viral replication in cell lines118,119,120, just this remains an experimental strategy rather than ane immediately amenable to clinical awarding. Screening of combinatorial chemic libraries has identified inhibitors of SARS protease, helicase and spike-protein–mediated prison cell entry121.
For successful treatment of influenza, antiviral drugs must exist administered inside 48 hours of disease onset to obtain substantial clinical result. But considering the SARS-CoV load increases until twenty-four hours ten of illness47, and in low-cal of the correlation of high viral load in the second week of disease with adverse outcomesixty, the window of opportunity for antiviral therapy may be wider.
Active and passive immunization
Much scientific effort has been focused on developing a vaccine to protect against future outbreaks of SARS-CoV. The commercial viability of developing a vaccine for SARS-CoV will ultimately depend on whether the virus re-emerges in the near hereafter. As discussed above, it is unlikely that future outbreaks will reach global proportions, just nevertheless, vaccines or passive immunization would exist relevant in the context of protecting high-run a risk individuals such equally laboratory and wellness-care workers. A vaccine could also exist considered in the setting of the farmed-game-animal trade, if farming of civets for human consumption continues. In the short fourth dimension since the virus was identified, substantial progress has been made toward developing a vaccine.
Immunodominant B- and T-cell epitopes of SARS-CoV are being divers122,123,124. Natural human infection with SARS-CoV leads to a long-lived neutralizing antibody response and immune sera crossneutralize various human SARS-CoV125, suggesting that agile immunization against SARS may be a feasible proposition. Simply and so far there has been no known case of human re-exposure to SARS-CoV to confirm that the naturally caused allowed response confers protection from reinfection. When SARS-CoV fasten, envelope, membrane and nucleocapsid proteins were individually expressed in an adulterate parainfluenza type 3 vector, only the recombinants expressing the spike protein induced neutralizing antibody and protected from claiming in hamsters102 (Table two). Mucosal immunization of African green monkeys with this parainfluenza–spike poly peptide chimeric virus led to neutralizing antibiotic and protection from viral replication in the upper and lower respiratory tracts after challenge with live SARS-CoV100, and spike protein–encoding Deoxyribonucleic acid vaccines stimulated neutralizing antibody product and protection from live-virus challenge in mice126. These studies confirm the assumption that the spike poly peptide is the ascendant protective antigen for SARS. Experiments using adoptive transfer and T-cell depletion showed that humoral amnesty alone tin can confer protection126. Other vaccine strategies accept included the utilise of naked DNA127,128,129, adenoviral vectors130 or modified vaccinia (Ankara)131 and inactivated whole virus132,133. Many investigators have optimized the codon usage of the gene target to improve expression. In summary, all vaccines based on the spike protein seem to induce neutralizing antibody responses, and those carrying nucleoprotein can induce nucleoprotein-specific jail cell-mediated amnesty. But thus far only 4 studies have used alive SARS-CoV to challenge immunized animals (Table 2). An inactivated vaccine with alum adjuvant, which induces neutralizing antibody in mice, is entering stage 1 human clinical trials in Mainland chinaxiii.
Experience with coronavirus vaccines for animals is relevant for SARS vaccine development134. Ane problem facing brute coronavirus vaccines has been strain variation among field isolates, leading to variable vaccine efficacy. A further concern is the experience with feline infectious peritonitis coronavirus, in which prior immunization led to enhanced disease rather than protection135. In the case of SARS-CoV, neither vaccination nor passive transfer of antibody has yet been reported to lead to disease enhancement, simply claiming with live SARS-CoV has occurred soon after immunization. Whether waning immunity or low titers of antibiotic lead to SARS disease enhancement remains unclear; the recent suggestion that immunized ferrets became more ill later on challenge clearly needs to be confirmed or refutedthirteen.
Passive transfer of immune serum protects naive mice from SARS-CoV infection101, and hyperimmune globulin with sufficient neutralizing activity for use in humans could be prepared from pooled ambulatory human plasma or from horses immunized with inactivated vaccine. Alternatively, monoclonal antibodies with sufficient neutralizing antibiotic activeness have been developed past screening phage-brandish antibody libraries and by immortalizing B-cell repertoires of convalescent SARS individuals with Epstein-Barr virus (Table 3)136,137,138. I of these (80R) blocks the virus–ACE-2 receptor interaction through bounden to the spike protein S1 domain136. Passive immunization of ferrets and mice was constructive in suppressing viral replication in lungs, but less so in the nasopharynx137,138. No randomized placebo control trial evaluated antibody therapy for pre- or mail service-exposure prophylaxis in at-risk groups during the SARS outbreak. Retrospective analysis of effect in a limited human study using human being SARS convalescent plasma suggested that passive immunization had no obvious adverse effects139.
The antigenic diversity of SARS-CoV-like precursor viruses in the wild-creature reservoir is undefined. In the event of a new interspecies manual event prompting another SARS outbreak, the crossprotection afforded past current vaccine constructs based on the human being SARS-CoV of 2003 is unknown and is likely to influence the efficacy of both passive and active immunization strategies.
Lessons learned
SARS provided a painful reminder of the global impact of emerging infectious diseases. Information technology illustrated how microbes, with their evolutionary drive to preserve and propagate their genes, exploit new opportunities and niches created past modern club140. Interspecies transmission of viruses to humans clearly has occurred throughout human history. But recent developments immune SARS-CoV increased opportunity to adjust to human-to-human transmission and, subsequently, to spread globally. In particular, big centralized wet markets and hospitals proved to be venues for distension of transmission to humans, and the burgeoning increment of international travel (currently ∼700 million travelers annually) exploded the local outbreak of an emerging infection into a potential pandemic.
Because near contempo emerging infectious disease threats take a zoonotic origin, we demand to better understand the microbial environmental of livestock and wildlife. In the context of increased attention and research funding directed at preparedness to gainsay bioterrorism threats, it is relevant to note that nature remains the greatest 'bioterrorist.' Although microbes that cause commercially of import diseases in livestock are well studied, organisms that pose threats to man health are non necessarily ones known to crusade disease in livestock, or for that matter, in wildlife. Nipah virus, Hendra virus and SARS-CoV all take a wild animals reservoir. Furthermore, now at that place is business organization over the possible role played by wild birds and ducks in the maintenance and spread of avian influenza A (H5N1) in Asia141. Greater understanding of the viral ecology of apparently good for you domestic animals and wildlife is therefore important. For example, the attention on ecological studies arising from the Nipah virus and SARS outbreaks have already led to the identification of a number of new viruses, including Tioman, Menangle, Australian bat lyssavirus and a novel group 1 coronavirus142,143. Some of these are now known to exist associated with human or livestock disease. But prioritizing such research efforts and assessing the public health relevance—if any—of such findings, poses challenges.
Three incidents of laboratory-caused SARS have arisen from biohazard level 3 and 4 laboratories, with community transmission arising from one (Globe Wellness Organization, http://www.wpro.who.int/sars/docs/update/update_07022004.asp). These incidents were associated with lapses in biohazard level 3 and iv practices. SARS-CoV tin can be safely handled in biohazard level three laboratories provided that biohazard level 3 practices are rigorously complied with. But every bit hospital health-care workers learned to their price, SARS-CoV is an unforgiving virus; one lapse may exist one also many, and it is irrelevant whether the lapse occurs in a biohazard level 3 or 4 laboratory.
Despite the impressive speed of scientific understanding of the disease, the global success in containing SARS owed much to traditional public wellness methods of clinical case identification, contact investigation, infection control at healthcare facilities, patient isolation and community containment (that is, quarantine)25. Only the awarding of such measures in modern club during the control of SARS highlighted several ethical and medical dilemmas, many of which arose from the need to balance individual freedoms confronting the common skillful144,145.
SARS signaled a paradigm shift in international public health. It highlighted the need for rapid information exchange regarding unusual communicable diseases outbreaks and the possibility of146, and the need for147, a coordinated global response to emerging infectious disease threats. During the early stages of the outbreak, the WHO acted independently, issuing travel alerts and geographically specific travel advisories, without the limited consent of the countries affected. The need for such measures was acknowledged post hoc by member states at the World Health Assembly meeting in May 2003 where the WHO was formally empowered to take such actions, as necessary, in the future. Although future emerging pandemics (due east.g., flu because of its transmissibility during early disease) may non be quelled through similar measures, the success of containing SARS remains a triumph for global public health.
References
-
Smith, Chiliad.D. Plague. in Manson's Tropical Diseases 21st edn (eds Cook, Chiliad.C. & Zumla, A.) Ch. 63, 1125–1131 (W. B. Saunders, London, 2003).
-
Shortridge, K.F. & Stuart-Harris, C.H. An influenza epicentre? Lancet 2, 812–813 (1982).
-
Centers for Affliction Control and Prevention. Update: outbreak of severe acute respiratory syndrome—worldwide, 2003. Morbid. Mortal. Wkly. Rpt. 52, 241–248 (2003).
-
Tsang, Grand.Due west. et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. Due north. Engl. J. Med. 348, 1977–1985 (2003).
-
Lee, N. et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 348, 1986–1994 (2003).
-
Poutanen, S.G. et al. Identification of severe astute respiratory syndrome in Canada. Northward. Engl. J. Med. 348, 1995–2005 (2003).
-
Peiris, J.South. et al. Coronavirus as a possible cause of severe astute respiratory syndrome. Lancet 361, 1319–1325 (2003).
-
Ksiazek, T.G. et al. A novel coronavirus associated with severe astute respiratory syndrome. N. Engl. J. Med. 348, 1953–1966 (2003).
-
Drosten, C. et al. Identification of a novel coronavirus in patients with astringent acute respiratory syndrome. Due north. Engl. J. Med. 348, 1967–1976 (2003).
-
Poon, L.Fifty. et al. Rapid diagnosis of a coronavirus associated with severe acute respiratory syndrome (SARS). Clin. Chem. 49, 953–955 (2003).
-
Marra, M.A. et al. The genome sequence of the SARS associated coronavirus. Science 300, 1399–1404 (2003).
-
Rota, P.A. et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300, 1394–1399 (2003).
-
Enserink, M. One year after outbreak. SARS virus yields some secrets. Science 304, 1097 (2004).
-
Peiris, J.S.Thou., Yuen, K.Y., Osterhaus, A.D.M.East. & Stohr, M. The severe astute respiratory syndrome. N. Engl. J. Med. 349, 2431–2441 (2003).
-
Christian, G.D., Poutanen, S.One thousand., Loufty, M.R., Muller, 1000.P. & Low, D.E. Severe astute respiratory syndrome. Clin. Infect. Dis. 38, 1420–1427 (2004).
-
Rainer, T.H. Severe acute respiratory syndrome: clinical features, diagnosis and management. Curr. Opin. Pulm. Med. 10, 159–165 (2004).
-
Drosten, C., Preiser, W., Gunther, S., Schmitz, H. & Doerr, H.W. Severe astute respiratory syndrome: identification of the aetiologic agent. Trends Mol. Med. 9, 325–327 (2003).
-
Holmes, Yard.Five. SARS coronavirus: a new claiming for prevention and therapy. J. Clin. Invest. 111, 1605–1609 (2003).
-
Davidson, A. & Siddell, S. Potential for antiviral handling of astringent astute respiratory syndrome. Curr. Opin. Infect. Dis. 26, 565–571 (2003).
-
Stadler, G. et al. SARS—start to understand a new virus. Nat. Rev. Microbiol. 1, 209–218 (2003).
-
Poon, L.L.Thousand., Guan, Y., Nicholls, N.J., Yuen, 1000.Y. & Peiris, J.Southward.M. The aetiology, origins and diagnosis of SARS. Lancet Infect. Dis. 4, 663–671 (2004).
-
Anderson, R.K. et al. Epidemiology, manual dynamics, and control of SARS. The 2002–2003 epidemic. Phil. Trans. R. Soc. Lond. B 359, 1091–1105 (2004).
-
Poutanen, S.One thousand. & Depression, D.E. Severe acute respiratory syndrome: an update. Curr. Opin. Infect. Dis. 17, 287–294 (2004)
-
Poutanen, S.1000. & McGeer, A.J. Transmission and control of astringent acute respiratory syndrome. Curr. Infect. Dis. Rep. 6, 220–227 (2004)
-
Weinstein, R.A. Planning for epidemics—the lessons of SARS. Due north. Engl. J. Med. 350, 2332–2334 (2004).
-
Zhong, North.Southward. et al. Epidemiology and crusade of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of People's republic of china, in February 2003. Lancet 362, 1355–1358 (2003).
-
Xu, R.H. et al. Epidemiologic clues to SARS origin in Red china. Emerg. Infect. Dis. 10, 1030–1037 (2004).
-
Zhong, Due north.S. & Zeng, K.Q. Our strategies for fighting severe astute respiratory syndrome (SARS). Am. J. Respir. Crit. Intendance Med. 168, vii–9 (2003).
-
Guan, Y. et al. Isolation and label of viruses related to SARS coronavirus from animals in southern China. Science 302, 276–278 (2003).
-
Centers for Affliction Control and Prevention. Prevalence of IgG antibody to SARS-associated coronavirus in brute traders—Guangdong Province, China, 2003. Morb. Mortal. Wkly. Rep. 52, 986–987 (2003).
-
Klempner, M.Southward. & Shapiro, D.S. Crossing the species barrier—one small footstep to man, 1 giant leap to mankind. Due north. Engl. J. Med. 350, 1171–1172 (2004).
-
Snijder, East.J. et al. Unique and conserved features of genomes and proteome of SARS-coronavirus, an early split up-off from the coronavirus group 2 lineage. J. Mol. Biol. 33, 991–1004 (2003).
-
Gorbalenya, A.E., Snijder, A.E. & Spaan, W.J. Severe astute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 78, 7863–7866 (2004).
-
The Chinese SARS Molecular Epidemiology Consortium. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in Red china. Science 303, 1666–1669 (2004).
-
Guan, Y. et al. Molecular epidemiology of SARS coronavirus in Hong Kong. Lancet 363, 99–104 (2004).
-
Ruan, Y. et al. Comparative full-length genome sequence analysis of fourteen SARS coronavirus isolates and mutual mutations associated with putative origins of infection. Lancet 361, 1779–1785 (2003).
-
Yeh, S.H. et al. Characterization of severe acute respiratory syndrome coronavirus genomes in Taiwan: molecular epidemiology and genome evolution. Proc. Natl. Acad. Sci. USA 101, 2542–2547 (2004).
-
Zhong, N. Management and prevention of SARS in China. Phil. Trans. R. Soc. Lond. B. 359, 1115–1116 (2004).
-
Liang, G. et al. Laboratory diagnosis of 4 recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg. Infect. Dis. 10, 1774–1781 (2004).
-
Wong, T.W. et al. Cluster of SARS amongst medical students exposed to single patient, Hong Kong. Emerg. Infect. Dis. x, 269–276 (2004).
-
Seto, West.H., et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of astringent acute respiratory syndrome (SARS). Lancet 361, 1519–1520 (2003).
-
Loon, South.C. et al. The severe acute respiratory syndrome coronavirus in tears. Br. J. Ophthalmol. 88, 861–863 (2004).
-
Leung, G.Yard. et al. SARS CoV antibody prevalence in all Hong Kong patient contacts. Emerg. Infect. Dis. ten, 1653–1656 (2004)
-
Lipsitch, M. et al. Transmission dynamics and control of astringent astute respiratory syndrome. Science 300, 1966–1970 (2003).
-
Goh, D.L.Yard. et al. Secondary household transmission of SARS, Singapore. Emerg. Infect. Dis. x, 232–234 (2004).
-
Shen, Z. et al. Super-spreading SARS events in Beijing, 2003. Emerg. Infect. Dis. ten, 256–260 (2004).
-
Peiris, J.Southward. et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361, 1767–1772 (2003).
-
Chan, K.H. et al. Detection of SARS coronavirus (SCoV) by RT-PCR, civilisation, and serology in patients with acute respiratory syndrome (SARS). Emerg. Infect. Dis. ten, 294–299 (2004).
-
Chan, P.K. et al. Laboratory diagnosis of SARS. Emerg. Infect. Dis. 10, 825–830 (2004).
-
Li, Fifty. et al. SARS-coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J. Clin. Virol. 28, 239–244 (2003).
-
Ng, E.Thousand. et al. Serial analysis of the plasma concentration of SARS coronavirus RNA in pediatric patients with severe acute respiratory syndrome. Clin. Chem. 49, 2085–2088 (2003).
-
Wong, R.S.One thousand. et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 326, 1358–1362 (2003).
-
Li, T. et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 189, 648–651 (2004).
-
Leung, C.W. et al. Astringent acute respiratory syndrome in children. Pediatrics 113, e535–e543 (2004).
-
Hon, K.L. et al. Clinical presentation and outcome of severe acute respiratory syndrome in children. Lancet 361, 1701–1703 (2003).
-
Drosten, C. et al. Evaluation of advanced opposite transcription PCR assays and an alternative PCR target region for detection of severe astute respiratory syndrome-associated coronavirus. J. Clin. Microbiol. 42, 2043–2047 (2004).
-
Cheng, P.K.C. et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet 363, 1699–1700 (2004).
-
Kaiser, L., Briones, M.Due south. & Hayden, F.G. Performance of virus isolation and Directigen Flu A to detect influenza A virus in experimental human infection. J. Clin. Virol. 14, 191–197 (1999).
-
Ng, Eastward.K. et al. Quantitation analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe astute respiratory syndrome. Clin. Chem. 49, 1976–1980 (2003).
-
Hung, I.F. et al. Viral loads in clinical specimens and SARS manifestations. Emerg. Infect. Dis. 10, 1550–1557 (2004).
-
Chu, C.M. et al. The role of lopinavir/ritonavir in treatment of SARS: initial virological and clinical findings. Thorax 59, 252–256 (2004).
-
Mazzulli, T. et al. Severe astute respiratory syndrome–associated coronavirus in lung tissue. Emerg. Infect. Dis. ten, 20–24 (2003).
-
Nicholls, J.M. et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet 361, 1773–1778 (2003).
-
Franks, T.J. et al. Lung pathology of astringent acute respiratory syndrome (SARS): a study of 8 dissection cases from Singapore. Hum. Pathol. 34, 743–748 (2003).
-
Chow, Yard.C. et al. Detection of severe acute respiratory syndrome-associated coronavirus in pneumocytes of the lung. Am. J. Clin. Pathol. 121, 574–580 (2004).
-
To, K.F. et al. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in situ hybridization report of fatal cases. J. Pathol. 202, 157–163 (2004).
-
Nakajima, N. et al. SARS Coronavirus-infected cells in lung detected by new in situ hybridization technique. Jpn. J. Infect. Dis. 56, 139–141 (2003).
-
Chong, P.Y. et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore. Arch. Pathol. Lab. Med. 128, 195–204 (2004).
-
Ding, Y. et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS CoV) in SARS patients: implications for pathogenesis and virus manual pathways. J. Pathol. 203, 622–630 (2004).
-
To, K.F. & Lo, A.Due west.I. Exploring the pathogenesis of severe astute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS CoV) and its putative receptor, angiotensin-converting-enzyme 2 (ACE-2). J. Pathol. 203, 740–743 (2004).
-
Leung, W.Thousand. et al. Enteric interest of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125, 1011–1017 (2003).
-
Chan, P.M.S. et al. Persistent infection of SARS coronavirus in colonic cells in vitro. J. Med. Virol. 74, ane–7 (2004).
-
Cinatl, J. et al. Infection of cultured intestinal epithelial cells with severe astute respiratory syndrome coronavirus. Jail cell Mol. Life Sci. 61, 2100–2112 (2004).
-
Yang, Z.Y. et al. pH-dependent entry of astringent astute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic prison cell transfer through DC SIGN. J. Virol. 78, 5642–5650 (2004).
-
Simmons, G. et al. Characterization of severe astute respiratory syndrome-associated coronavirus (SARS CoV) fasten glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U.s. 101, 4240–4245 (2004).
-
Hoffmann, H. et al. Susceptibility to SARS coronavirus S protein-derived infection correlates with expression of angiotensin converting enzyme two and infection tin be blocked by soluble receptor. Biochem. Biophys. Res. Comm. 319, 1216–1221 (2004).
-
Han, D.P., Kim, H.Chiliad., Kim, Y.B., Poon, L.L.M. & Cho, M.W. Evolution of a rubber neutralization assay for SARS CoV and characterization of Due south-glycoprotein. Virology 326, 140–149 (2004).
-
Bosch, B.J. et al. Astringent astute respiratory syndrome associated coronavirus (SARS CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 101, 8455–8460 (2004).
-
Liu, S. et al. Interaction between heptad repeat i and ii regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechnism and identification of fusion inhibitors. Lancet 363, 938–947 (2004).
-
Qinfen, Z., et al. The life bike of SARS coronavirus in Vero E6 cells. J. Med. Virol. 73, 332–337 (2004).
-
Li, W. et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
-
Wang, P. et al. Expression cloning of functional receptor used by SARS coronavirus. Biochem. Biophys. Res. Commun. 315, 439–444 (2004).
-
Babcock, Grand.J., Esshaki, D.J., Thomas, W.D. & Ambrosino, D.Yard. Amino acids 270 to 510 of the severe astute respiratory syndrome spike poly peptide are required for interaction with receptor. J. Virol. 78, 4552–4560 (2004).
-
Lai, 1000.G.C. & Holmes, 1000.V. Coronaviridae and their replication. in Fields' Virology Vol two. (eds Knipe, D.Thou. & Howley, P.M) Ch. 35, 1163–1185 (Lippincott Williams & Wilkins, Philadelphia, Pennsylvania, 2001).
-
Hammling, I. et al. Tissue distribution of ACE-2 protein, the functional receptor for SARS coronavirus. A beginning footstep in understanding SARS pathogenesis. J. Pathol. 203, 631–637 (2004).
-
Yount, B. et al. Reverse genetics with a full-length infectious cDNA of astringent acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 100, 12995–13000 (2003).
-
Wong, C.Thou. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136, 95–103 (2004).
-
Zhang, Y. et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 72, 4410–4415 (2004).
-
Ng, P.C. et al. Inflammatory cytokine profile in children with severe astute respiratory syndrome. Pediatrics 113, e7–e14 (2004).
-
Lee, C.H. et al. Altered p38 mitogen-activated protein kinase expression in different leukocytes with increase of immunosuppressive mediators in patients with astringent astute respiratory syndrome. J. Immunol. 172, 7841–7847 (2004).
-
Peiris, J.S.Chiliad. et al. Re-emergence of fatal human influenza A subtype H5N1 illness. Lancet 363, 617–619 (2004).
-
Jones, B.Thousand. et al. Prolonged disturbance of in vitro cytokine production inpatients with severe acute respiratory syndrome (SARS) treated with ribavirin and steroids. Clin. Exp. Immunol. 135, 467–473 (2004).
-
Openshaw, P.J.M. What does the peripheral blood tell you in SARS? Clin. Exp. Immunol. 136, 11–12 (2004).
-
Lin, Thousand. et al. Clan of HLA form ane with severe acute respiratory syndrome coronavirus infection. BMC Med. Genet. iv, 9 (2003).
-
Ng, Chiliad.H.L. et al. Association of human-leukocyte-antigen class 1 (B*0703) and class Ii (DRB1*0301) genotypes with susceptibility and resistance to the development of astringent acute respiratory syndrome. J. Infect. Dis. 190, 5151–5158 (2004).
-
Fouchier, R.A.K. et al. Koch's postulates fulfilled for SARS virus. Nature 423, 240 (2003).
-
Kuiken, T. et al. Newly discovered coronavirus as the primary crusade of severe acute respiratory syndrome. Lancet 362, 263–270 (2003).
-
Rowe, T. et al. Macaque model for severe acute respiratory syndrome. J. Virol. 78, 11410–11414 (2004).
-
Martina, B.E. et al. Virology: SARS virus infection of cats and ferrets. Nature 425, 915 (2003).
-
Bukreyev, A. et al. Mucosal immunisation of African green monkeys (Cercopithicus aethiops) with an adulterate parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet 363, 2122–2127 (2004).
-
Subbarao, K. et al. Prior infection and passive transfer of neutralizing antibody preclude replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 78, 3572–3577 (2004).
-
Buchholtz, U.J. et al. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective amnesty. Proc. Natl. Acad. Sci. USA 101, 9804–9809 (2004).
-
Roberts, A. et al. SARS coronavirus infection in Gilt Syrian hamsters. J. Virol. (in the press).
-
Wu, C.Y. et al. Minor molecules targeting astringent acute respiratory syndrome human being coronavirus. Proc. Natl. Acad. Sci. USA 101, 10012–10017 (2004).
-
Wu, C.J. et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 48, 2693–2696 (2004)
-
Chen, F. et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 31, 69–75 (2004).
-
Yi, Y. et al. Small molecules blocking the entry of severe astute respiratory syndrome coronavirus into host cells. J. Virol. 78, 11334–11339 (2004).
-
Yamamoto, Northward. et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 318, 719–725 (2004).
-
Tan, Due east.Fifty. et al. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. x, 581–586 (2004).
-
Hensley, L.E. et al. Interferon-β 1a and SARS coronavirus replication. Emerg. Infect. Dis. ten, 317–319 (2004).
-
Stroher, U. et al. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J. Infect. Dis. 189, 1164–1167 (2004).
-
Spiegel, M., Pichlmair, A., Muhlberger, E., Haller, O. & Weber, F. The antiviral result of interferon-β against SARS coronavirus is not mediated by MxA protein. J. Clin. Virol. 30, 211–213 (2004).
-
Zhang, X.West. & Yap, Y.L. Old drugs as lead compounds for a new disease? Binding assay of SARS coronavirus principal proteinase with HIV, psychotic, and parasitic drugs. Bioorg. Med. Chem. 12, 2517–2521 (2004).
-
Haagmans, B.L. et al. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. ten, 290–293 (2004).
-
Loutfy, Thou.R. et al. Interferon alfacon-ane plus corticosteroids in astringent astute respiratory syndrome: a preliminary study. J. Am. Med. Assoc. 290, 3222–3228 (2003).
-
Anand, K. et al. Coronavirus primary protease (3Clpro) construction: footing for blueprint of antiviral drugs. Science 300, 1763–1767 (2003).
-
Ivanov, K.A. et al. Multiple enzymatic activities associated with astringent astute respiratory syndrome coronavirus helicase. J. Virol. 78, 5619–5632 (2004).
-
He, Yard.Fifty. et al. Inhibition of SARS-associated coronavirus infection and replication past RNA interference. JAMA 290, 2665–2666 (2003).
-
Wang, Z. et al. Inhibition of severe acute respiratory syndrome virus replication past small interfering RNA in mammalian cells. J. Virol. 78, 7523–7527 (2004).
-
Zhang, Y. et al. Silencing SARS CoV spike poly peptide expression in cultured cells by RNA interference. FEBS Lett. 560, 141–146 (2004).
-
Kao, R.Y. et al. Identification of novel minor molecule inhibitors of severe acute respiratory syndrome associated coronavirus by chemical genetics. Chem. Biol. 11, 1293–1299 (2004).
-
Lu, L. et al. Immunological characterization of the spike poly peptide of the severe astute respiratory syndrome coronavirus. J. Clin. Microbiol. 42, 1570–1576 (2004).
-
Zhang, H. et al. Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike protein capable of inducing neutralizing antibodies. J. Virol. 78, 6938–6945 (2004).
-
Wang, Y.D. et al. T jail cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike poly peptide elicit a specific T-cell immune response in patients who recover from SARS. J. Virol. 78, 5612–5618 (2004).
-
Nie, Y. et al. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J. Infect. Dis. 190, 1119–1126 (2004).
-
Yang, Z.Y. et al. A Deoxyribonucleic acid vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 428, 561–564 (2004).
-
Kim, T.W. et al. Generation and characterization of Deoxyribonucleic acid vaccines targeting the nucleocapsid protein of severe astute respiratory syndrome coronavirus. J. Virol. 78, 4638–4645 (2004).
-
Zeng, F. et al. Label of humoral responses in mice immunized with plasmid DNAs encoding SARS-CoV spike cistron fragments. Biochem. Biophys. Res. Commun. 315, 1134–1139 (2004).
-
Zhu, G.Southward. et al. Induction of SARS-nucleoprotein-specific immune response by apply of Deoxyribonucleic acid vaccine. Immunol Lett. 92, 237–243 (2004).
-
Gao, W. et al. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet 362, 1895–1896 (2003).
-
Bisht, H. et al. Severe astute respiratory syndrome coronavirus fasten protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. USA 101, 6641–6646 (2004).
-
Takasuka, N. et al. A subcutaneously injected UV-inactivated SARS coronavirus vaccine elicits systemic humoral immunity in mice. Int. Immunol. 16, 1423–1430 (2004).
-
Tang, L. et al. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 23, 391–394 (2004).
-
Cavanagh, D. Severe acute respiratory syndrome vaccine evolution: experiences of vaccination confronting avian infectious bronchitis coronavirus. Avian Pathol. 32, 567–582 (2003).
-
Olsen, C.W. A review of feline infectious peritonitis virus: molecular biology, immunopathogenesis, clinical aspects, and vaccination. Vet. Microbiol. 36, i–37 (1993).
-
Sui, J. et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAB to S1 protein that blocks receptor clan. Proc. Natl. Acad. Sci. USA 101, 2536–2641 (2004).
-
Ter Muelen, J. et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet 363, 2139–2141 (2004).
-
Traggian, East. et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 10, 871–875 (2004).
-
Soo, Y.O.Y. et al. Retrospective comparison of convalescent plasma with continuing high dose methyl prednisolone handling in SARS patients. Clin. Microbiol. Infect. 10, 657–658 (2004)
-
Smolinski, M.S., Hamburg, K.A. & Lederberg, J. (eds). Microbial Threats to Health: Emergence, Detection and Response (National Academy Press, Washington, DC, 2003).
-
Li, Thou.Due south. et al. Genesis of a highly pathogenic and potentially pandemic H5N1 flu virus in eastern Asia. Nature 430, 209–213 (2004).
-
Mackenzie, J.Southward., Field, H.F. & Guyatt, Thousand.J. Managing emerging diseases borne by fruit bats (flying foxes), with detail reference to henipaviruses and Australian bat lyssavirus. J. Appl. Microbiol. 94 Suppl, 59S–69S (2003).
-
Poon, L.50.Yard. et al. Identification of a novel coronavirus in bats. J. Virol. (in the press).
-
Singer, P.A. et al. Ethics and SARS: Lessons from Toronto. BMJ 327, 1342–1344 (2003).
-
O'Neill, O. Informed consent and public health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1133–1136 (2004)
-
World Health Organization. A multicentre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet 361, 1730–1733 (2003).
-
Fidler, D.P. Germs, governance, and global public health in the wake of SARS. J. Clin. Invest. 113, 799–804 (2004).
Acknowledgements
We thank K.Five. Holmes, L.L.M. Poon and J.M. Nicholls for critical comment on the manuscript; A. Frazier for scientific editing; and F. Wong for secretarial assist. We acknowledge enquiry funding from the United States National Institutes of Wellness (grant AI95357), the Wellcome Trust (grant 067072/D/02/Z) and the Research Fund for the Command of Infectious Diseases from the Government of Hong Kong Special Administrative Region.
Author information
Affiliations
Respective author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
Most this article
Cite this commodity
Peiris, J., Guan, Y. & Yuen, Thousand. Severe acute respiratory syndrome. Nat Med 10, S88–S97 (2004). https://doi.org/10.1038/nm1143
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/nm1143
Further reading
Source: https://www.nature.com/articles/nm1143